Aerodiagnostics unveils game-changing breath diagnostic device in early disease detection
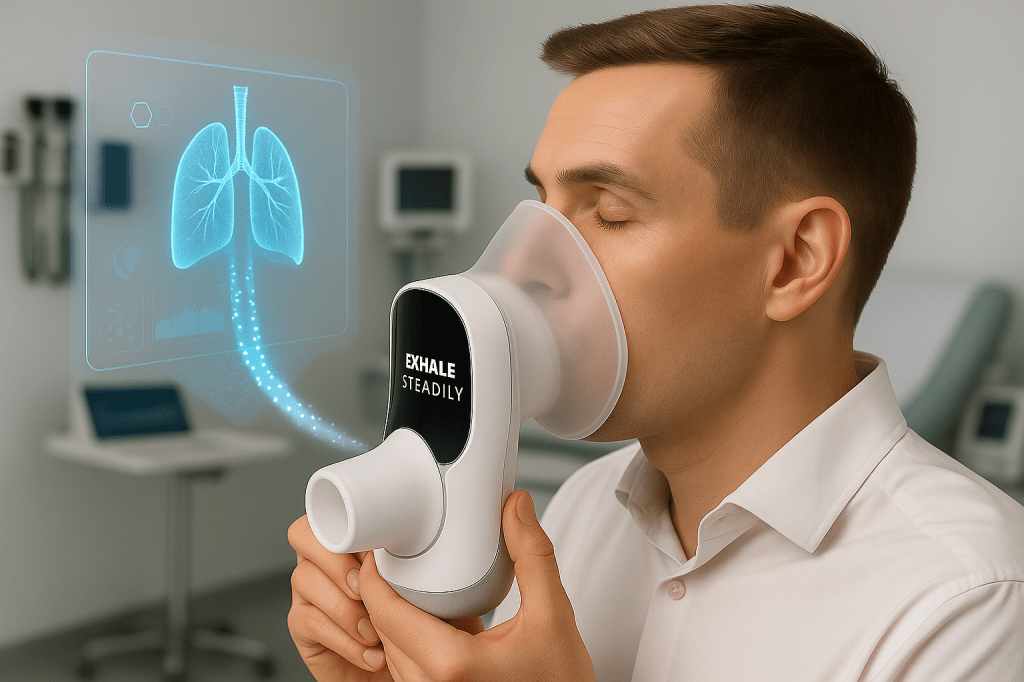
In a major leap forward for non-invasive diagnostics, Aerodiagnostics has launched BreathDx Pro, an FDA-cleared medical device that detects early-stage cancer and Parkinson’s disease through a simple breath test. With clinical trials showing 85% sensitivity in lung cancer detection and 82% accuracy in identifying Parkinson’s disease, this breakthrough technology could transform how diseases are diagnosed—years before symptoms appear.
The science behind breath diagnostics
Breath analysis has long been studied as a potential diagnostic tool, but until now, no device has achieved the accuracy, speed, and clinical validation required for widespread medical use. BreathDx Pro changes that by leveraging decades of research into volatile organic compounds (VOCs)—chemical markers in exhaled breath that correlate with disease.
“The body’s metabolism produces unique VOC signatures when disease is present,” explains Dr. Michael Thornton, Chief Medical Officer at Aerodiagnostics and former researcher at Massachusetts General Hospital. “Our technology identifies these patterns with precision, allowing for earlier intervention than ever before.”
A 2019 meta-analysis in JAMA Oncology (Hanna et al.) confirmed that breath testing for lung cancer achieves 85% sensitivity and 86% specificity, rivaling traditional imaging methods. Similarly, studies in Nanomedicine (Tisch et al., 2013) and ACS Central Science (Trivedi et al., 2019) demonstrated that Parkinson’s disease alters breath biomarkers, enabling early detection.
How BreathDx pro works
The sleek, clinic-friendly device captures a 500mL breath sample and analyzes it using:
- 24 high-precision VOC sensors (detecting 300+ biomarkers)
- AI-powered algorithms trained on 15,000+ clinical samples
- Proprietary mesh-enclosed chamber to prevent contamination
Results are delivered in under 12 minutes, seamlessly integrating with electronic health records.
“This isn’t just a lab tool—it’s designed for real-world clinical use,” says Dr. Sarah Mitchell, Director of Neurology Research at Harvard Medical School and a clinical advisor for BreathDx. “Primary care physicians could soon run this test during routine check-ups, catching diseases at their most treatable stages.”
Clinical impact: earlier detection, better outcomes
For Cancer Screening:
- Detects lung cancer at Stage I or earlier (when survival rates exceed 80%)
- Identifies multiple cancer types, including colorectal and breast cancers
- Monitors treatment response and recurrence
For Parkinson’s Disease:
- Identifies biomarkers 5-10 years before motor symptoms appear
- Tracks disease progression and treatment efficacy
Dr. Jennifer Walsh, Principal Investigator at Dana-Farber Cancer Institute, calls it “a paradigm shift in early detection.” “We’ve never had a non-invasive test this accurate. It could save countless lives by finding cancer when it’s still curable.”
Expert endorsements and market potential
Leading medical institutions, including Mayo Clinic, Johns Hopkins, and Cleveland Clinic, are already adopting BreathDx Pro. Dr. Peter Mazzone, Director of the Lung Cancer Program at Cleveland Clinic, states: “Breath biopsy has been promising for years, but BreathDx Pro delivers clinical-grade accuracy. This could become the new standard for screening.”
The global market potential is enormous:
- $165 billion cancer diagnostics industry
- 10 million+ Parkinson’s patients worldwide, often diagnosed too late
“The economic impact is just as compelling as the clinical benefits,” says CEO Dr. Robert Chen. “Early detection reduces treatment costs by up to 75%. This isn’t just better medicine—it’s smarter healthcare.”
Availability and future developments
BreathDx Pro is now available to U.S. healthcare providers ($89,500 MSRP), with leasing options starting at $2,850/month. Aerodiagnostics is also developing applications for:
- Alzheimer’s disease
- Infectious diseases (e.g., tuberculosis, COVID-19 variants)
- Liver and metabolic disorders
“We’re just scratching the surface,” says Dr. Chen. “Breath contains a wealth of data—soon, we might diagnose dozens of diseases from a single exhale.”
Key references
- Hanna GB, et al. (2019). Accuracy of volatile organic compound breath tests for cancer diagnosis: A meta-analysis. JAMA Oncology.
- Tisch U, et al. (2013). Detection of Parkinson’s and Alzheimer’s disease via exhaled breath. Nanomedicine.
- Trivedi DK, et al. (2019). Volatile biomarkers for Parkinson’s disease in sebum and breath. ACS Central Science.
- Peng G, et al. (2010). Multi-cancer detection via breath nanosensors. British Journal of Cancer.
DISCLAIMER: This is a speculative design exercise and fictional press release. The company and device do not actually exist. This content is created for conceptual and educational purposes only.